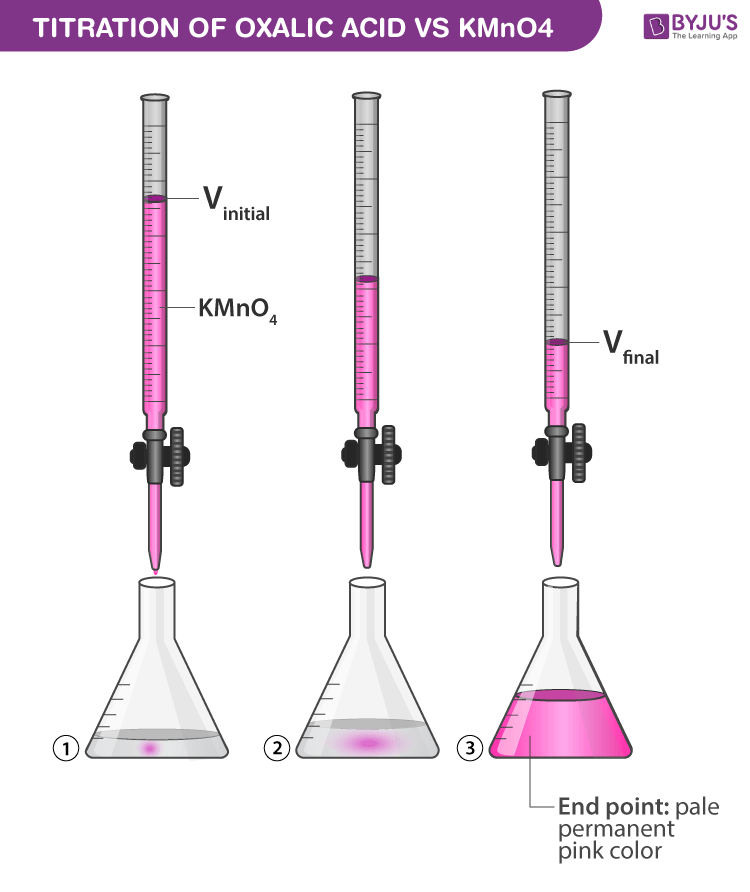
Olabs Titration Class 12 . titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). repeat the titration until concordant values are obtained. The readings are recorded in a tabular form as shown and. The labs designed for classes 9 to 12 are aligned with the ncert and scert. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Use the virtual laboratory to standardize an unknown. standardization of naoh with a khp solution: in this animated video, you will learn titration of kmno4 with mohr's salt.
from byjus.com
the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). The readings are recorded in a tabular form as shown and. standardization of naoh with a khp solution: in this animated video, you will learn titration of kmno4 with mohr's salt. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. repeat the titration until concordant values are obtained. The labs designed for classes 9 to 12 are aligned with the ncert and scert. Use the virtual laboratory to standardize an unknown.
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12
Olabs Titration Class 12 in this animated video, you will learn titration of kmno4 with mohr's salt. repeat the titration until concordant values are obtained. The readings are recorded in a tabular form as shown and. The labs designed for classes 9 to 12 are aligned with the ncert and scert. titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). Use the virtual laboratory to standardize an unknown. standardization of naoh with a khp solution: in this animated video, you will learn titration of kmno4 with mohr's salt. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration.
From dxompffry.blob.core.windows.net
Titration Formula Class 12 at Donald Rios blog Olabs Titration Class 12 The readings are recorded in a tabular form as shown and. standardization of naoh with a khp solution: the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. in this animated video, you will learn titration of kmno4 with mohr's salt. Use the virtual laboratory to standardize an unknown. The labs. Olabs Titration Class 12.
From www.youtube.com
How to prepare KMnO4 solution for titration of class xii calculation Olabs Titration Class 12 The labs designed for classes 9 to 12 are aligned with the ncert and scert. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. standardization of naoh with a khp solution: The readings are recorded in a tabular form as shown and. in this animated video, you will learn. Olabs Titration Class 12.
From www.youtube.com
Grade 12 Chemistry Lab Titrations of Weak Acids and Bases YouTube Olabs Titration Class 12 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). The labs designed for classes 9 to 12 are aligned with the ncert and scert. Use the virtual laboratory. Olabs Titration Class 12.
From www.studypool.com
SOLUTION Chemistry class xii record 22 23 titration and salt analysis Olabs Titration Class 12 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. repeat the titration until concordant values are obtained. standardization of naoh with a khp solution: the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. chemistry practical. Olabs Titration Class 12.
From www.youtube.com
️ Titration Experiment for Board Class Complete Video to Understand Olabs Titration Class 12 Use the virtual laboratory to standardize an unknown. The readings are recorded in a tabular form as shown and. titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). The labs designed for classes 9 to 12 are aligned with the ncert and scert. standardization. Olabs Titration Class 12.
From www.youtube.com
Titration class XII chemistry practical YouTube Olabs Titration Class 12 titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). standardization of naoh with a khp solution: repeat the titration until concordant values are obtained. Use the virtual laboratory to standardize an unknown. the titration of potassium permanganate (kmno 4) against oxalic acid. Olabs Titration Class 12.
From www.youtube.com
TITRATION CLASS12th BOARD PRACTICAL YouTube Olabs Titration Class 12 titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). The readings are recorded in a tabular form as shown and. standardization of naoh with a khp solution: in this animated video, you will learn titration of kmno4 with mohr's salt. the titration. Olabs Titration Class 12.
From www.youtube.com
chemistry practical class 12 experiment 1 Quantitative estimation Olabs Titration Class 12 titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. repeat the titration until concordant values are obtained. chemistry practical class 12 determination of concentration/molarity of kmno4. Olabs Titration Class 12.
From www.studocu.com
Class XII Chemistry 202223 Experiment No Oxidation Reduction Olabs Titration Class 12 the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. in this animated video, you will learn titration of kmno4 with mohr's salt. repeat the titration until concordant values are obtained. titration is a common laboratory method of qualitative chemical analysis that can be. Olabs Titration Class 12.
From www.youtube.com
KMnO4 VS Mohr’s Salt Titration, Class 12 by Kanchan Handa YouTube Olabs Titration Class 12 chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. The labs designed for classes 9 to 12 are aligned with the ncert and scert. standardization of naoh with a khp solution: Use the virtual laboratory to standardize an unknown. titration is a common laboratory method of qualitative chemical analysis. Olabs Titration Class 12.
From boardtopper.blogspot.com
Titration 2 Class 12 CBSE Practical All Study Guide at one Place! Olabs Titration Class 12 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. The labs designed for classes 9 to 12 are aligned with the ncert and scert. titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). repeat the titration. Olabs Titration Class 12.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Olabs Titration Class 12 The labs designed for classes 9 to 12 are aligned with the ncert and scert. The readings are recorded in a tabular form as shown and. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. in this animated video, you will learn titration of kmno4 with mohr's salt. titration. Olabs Titration Class 12.
From exogoqrkx.blob.core.windows.net
Titration Class 12Th Practical at Dolores Parker blog Olabs Titration Class 12 The readings are recorded in a tabular form as shown and. in this animated video, you will learn titration of kmno4 with mohr's salt. the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. repeat the titration until concordant values are obtained. the titration of potassium permanganate (kmno 4) against. Olabs Titration Class 12.
From dxompffry.blob.core.windows.net
Titration Formula Class 12 at Donald Rios blog Olabs Titration Class 12 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. standardization of naoh with a khp solution: The labs designed for classes 9 to 12 are aligned with the ncert and scert. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example. Olabs Titration Class 12.
From www.youtube.com
What is Titration in Chemistry Chemistry practical Class 12th Olabs Titration Class 12 repeat the titration until concordant values are obtained. the titration of potassium permanganate (kmno 4) against oxalic acid (c 2 h 2 o 4) is an example of redox titration. Use the virtual laboratory to standardize an unknown. in this animated video, you will learn titration of kmno4 with mohr's salt. The readings are recorded in a. Olabs Titration Class 12.
From www.youtube.com
How to Use Olabs / Online Lab Physics Class 12 Practical Ohm's Law Olabs Titration Class 12 The labs designed for classes 9 to 12 are aligned with the ncert and scert. titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). Use the virtual laboratory to standardize an unknown. in this animated video, you will learn titration of kmno4 with mohr's. Olabs Titration Class 12.
From www.youtube.com
OLABS Practical 1 Ohm's law and resistance Class 12 Physics YouTube Olabs Titration Class 12 the titration of potassium permanganate (kmno 4) against mohr salt is an example of redox titration. in this animated video, you will learn titration of kmno4 with mohr's salt. titration is a common laboratory method of qualitative chemical analysis that can be used to determine the unknown concentration of a solution (analyte). chemistry practical class 12. Olabs Titration Class 12.
From www.youtube.com
Volumetric Titration Demonstration Class XII YouTube Olabs Titration Class 12 The labs designed for classes 9 to 12 are aligned with the ncert and scert. chemistry practical class 12 determination of concentration/molarity of kmno4 solution by titrating it against a standard solution. repeat the titration until concordant values are obtained. in this animated video, you will learn titration of kmno4 with mohr's salt. titration is a. Olabs Titration Class 12.